What Makes the Active Chemistry® Curriculum Unique?
Active Chemistry® is a NSF-funded high school chemistry curriculum that embraces the three-dimensional learning of the Next Generation Science Standards for ALL students.
Available as a full-year high school chemistry curriculum or as chapter books for extended day and summer school programs.
Available as a package or for individual purchase
Active Chemistry® Print Teacher and Student Materials
Digital
Teacher and Student Interactive Digital Edition of Active Chemistry®
Kits
Active Chemistry® Materials & Supplies
Professional Learning
Personalized Services to Support Implementation
Three-Dimensional
Project-Based Approach
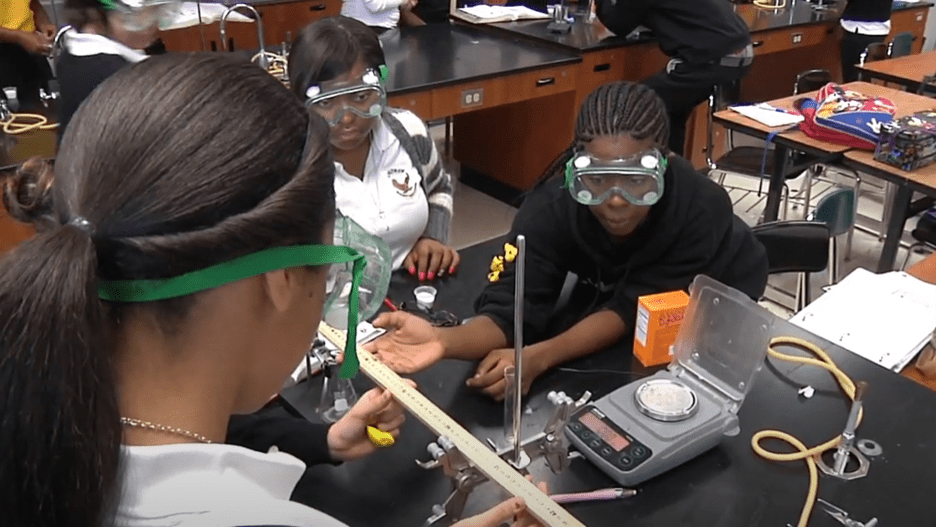
Students conduct investigations and engage in the Engineering Design Cycle as they iteratively work towards completing the Chapter Challenge.
Students Learn Like Scientists and Engineers
Students develop important
21st-Century skills as they work collaboratively in groups and engage in scientific discourse.
Total Support
for Teachers
Professional Learning is provided by our team of education experts.
Need more info to decide if this the right curriculum for your district or school?
Active Chemistry® Curriculum Details
Active Chemistry® fosters scientifically literate students who will be prepared for the workforce, able to make informed decisions, and contribute as productive citizens in the 21st century.
-
Chapter Challenges
Interesting and meaningful Chapter Challenges motivate students to learn and remember the chemistry content.
-
Crosscutting Concepts
Science and engineering practices, crosscutting concepts, and core ideas are seamlessly integrated throughout the Active Chemistry® curriculum.
-
Project-Based Active Series
Explore the project-based Active Series for Active Chemistry®, Physics, and Physical Science and our EarthComm Earth and Space System Science curriculum.
-
NGSS Alignment
Download the Active Chemistry® program brochure to view the detailed NGSS alignment.
Active Chemistry® Research-Based Design
The Active Chemistry® Curriculum is research-based.
Active Chemistry® was supported through National Science Foundation (NSF) funding and consequently produced through rigorous, iterative, research-based development cycles. It is based on the latest research from the cognitive sciences on how students learn.
Students develop communication and collaboration skills.
In the Active Chemistry® program, students develop a community of practice and a culture of collaboration, creativity, critical thinking, and communication.
The presentations of the Chapter Challenges provide students with opportunities to engage in scientific arguments using evidence and science knowledge and promote a deeper understanding through public practice.
Active Chemistry® fits your standards.
The Active Chemistry® curriculum reflects the full scope of chemistry content standards for high school, those identified as the Disciplinary Core Ideas in A Framework for K-12 Science Education, and those of individual states and districts.
Chapter Challenge: Students develop a movie scene that uses special effects which involve chemical concepts.
Through investigation, students identify compounds and elements and decompose water. They examine the states of matter and relate them to molecular motion. Then they observe the Tyndall effect and differentiate among solutions, suspensions, and colloids.
Students measure the volume and mass of liquids and solids to develop an understanding of density. They use flame tests to identify metal cations. They also learn about organic materials and use the law of conservation of mass to balance combustion reactions.
Digital Platform
The Activate Learning Digital Platform (ALDP) hosts the interactive digital edition of the Active Chemistry® teacher and student curriculum materials.
The platform is designed for student accessibility and inclusion and offers embedded translation for over 130 languages and text-to-speech with read-along highlighting in 35 languages.
Featuring an intuitive user experience, teachers have everything they need to Plan, Teach, Assign, and Assess lessons in a platform that is integrated with leading SIS rostering and Learning Management Systems such as Google Classroom, Schoology, and Canvas.
About The Author

Dr. Arthur Eisenkraft has taught high school physics for over 28 years. He is currently the Distinguished Professor of Science Education, Professor of Physics and Founding Director of the Center of Science and Math in Context (COSMIC) at the University of Massachusetts Boston.
Testimonials Hear Directly from Administrators & Teachers.
Active Chemistry® Videos
Active Chemistry® Overview

Active Chemistry® Classroom